Abstract
Introduction:There is an unmet need for rescue treatment of Non-Hodgkin Lymphoma (NHL) patients who are refractory to initial treatment or relapse after immuno-chemotherapy or after receiving a transplant.
Pixantrone is a novel aza-anthracenedione structurally different to anthracyclines and anthracenediones which is thought to be associated with reduced anthracycline-related cardiotoxicity due to a purported reduction in free-radical formation and cardiotoxic alcohols. Due to the lack of Real World Data in patients treated with pixantrone, we perform this study with the objective to evaluate the effectiveness and safety of the drug in a patient's population from Spain and Italy.
Methods: An observational retrospective registry of patients was designed. Data were collected from across all centres that have treated at least one patient with pixantrone (one cycle or more) in Spain and from selected centres in Italy.
Eligible patients were at least 18 years old, had histologically proven relapsed or refractory aggressive B-cell NHL and had to be treated according to pixantrone product information (SmPC).
Informed consent was obtained from patients still alive when the study was initiated. Pixantrone was administered as monotherapy (50 mg/m2 on days 1, 8 and 15 of a 28 days cycle).
Clinical baseline characteristics at the time of starting pixantrone were collected: age, gender, international prognostic index (IPI), Eastern Cooperative Oncology Group (ECOG) performance status, extranodal disease, B symptoms and Ann Arbor stage. Details on prior therapy before pixantrone were collected, including transplantation, radiotherapy, and chemotherapy. Type of regimen, duration, best Overall Response Rate (ORR) and rituximab use were registered for the last therapeutic treatment received. Pixantrone treatment specifications as treatment duration, dose delays and dose reductions, ECOG and best outcome were collected. Follow up data after pixantrone treatment and safety information were also requested.
Primary endpoint was the Progression Free Survival (PFS). Secondary endpoints included: the proportion of patients who reached a complete response (CR) after pixantrone treatment; ORR and OS.
This study is ongoing and recruiting patients in Spain and Italy.
Results: In this preliminary analysis 49 patients were included in 31 centers. It is expected to include 90 patients until final database closure.
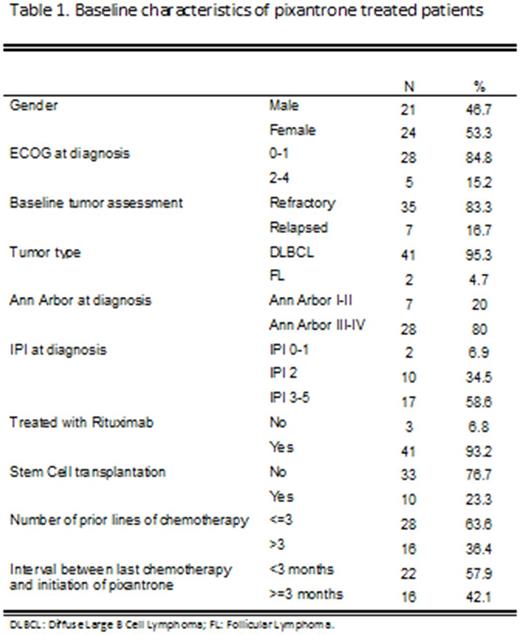
The key baseline characteristics of analyzed patients are outlined in Table 1. The mean age at diagnosis was 65 years (95% CI 61-70). The median number of cycles of treatment with pixantrone was 2 (range, 1-6). Twenty seven patients (58.7%) died in the follow-up up to date. Data on ORR, PFS, and OS will be detailed in the final report, expected in December, 2017.
Until now, related or possibly related to pixantrone adverse reactions (AR) were reported in 28 of 38 patients (73.7%). The most common reported adverse reactions were hematological (58.7%), being gastrointestinal adverse reactions the most common non-hematological toxicity reported (10.7%). and administration-site reactions (10.7%). Regarding the ARs severity, Grade 2 were the most frequently reported (36%), followed by Grade 3 (21.3%) and Grade 4 (18.6%).No cardiotoxicity has been reported.
Conclusion: These preliminary results of pixantrone treatment in real world clinical practice showed that pixantrone was well tolerated with no evidence of cardiac toxicity. The effectiveness of pixantrone in this study will be confirmed with the future final registry results.
Zinzani: Janssen: Consultancy; Lundbeck: Consultancy; Gilead Sciences: Consultancy; Roche: Consultancy; Celgene: Consultancy; Mundipharma: Consultancy; Takeda: Consultancy; Verastem: Consultancy; Sandoz: Consultancy; Bristol-Myers Squibb: Consultancy; Merck: Consultancy; Servier: Consultancy; Nordic Nanovector: Consultancy; Astellas: Consultancy. Navarro: Laboratorios Servier: Consultancy. Sancho: Celgene: Honoraria; Gilead: Honoraria; Laboratorios Servier: Consultancy; Janssen: Honoraria; Mundipharma: Honoraria; Roche: Honoraria; Kern Pharma: Honoraria. Soler: Laboratorios Servier: Consultancy. Grasso Cicala: Laboratorios Servier: Employment. Yapur: Laboratorios Servier: Employment. Spione: Servier Italy: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal